Assign modules on offcanvas module position to make them visible in the sidebar.
Main Menu
Sepcial Promotion
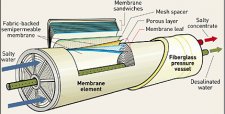
The nucleation and growth of inorganic scale on membrane surfaces is a problem shared by operators of reverse osmosis systems. While suite of indicators exists to predict scale formation based on pure compound solubility, it is apparent the onset of crystal nucleation and the properties of precipitated scale in reverse osmosis systems operating on natural waters differs from what would be expected in controlled conditions. Managing scale formation relies on understanding the chemistry of the scale, judicious system design, appropriate chemical application and early detection. The following review considers the mechanism of scale formation and the properties of alkaline, non-alkaline and silica based scales that are encountered when reverse osmosis is used in desalination, brackish water and wastewater recycling applications. Management of scale formation can be achieved at the design stage by the inclusion of unit processes to the scale forming constituents or by the application of antiscalants that delay the onset of nucleation. A range of conventional and emerging analytical techniques, including direct observation and spectroscopic methods have evolved to detect scale formation in real time. It is apparent, however, the type of scale encountered, limits of system recovery, process configurations and decisions on chemical selection are site specific. In particular, in wastewater reclamation, the high reporting incidence of calcium phosphate scale suggests that more research is needed on the mechanisms, control strategies for this scale. The paper highlights the need for molecular level understanding and various factors including the need for greater transparency of antiscalant formulations, studies on natural waters and complex water chemistry.